Brexpiprazole’s FDA Approval for Agitation in Dementia: Is it a Game Changer?
Clinically, not really... but perhaps bureaucratically?
On May 11, 2023, Rexulti (brexpiprazole) was approved by the FDA to treat agitation associated with dementia due to Alzheimer’s Disease. It’s the first FDA-approved treatment option for this indication, so it has deservedly generated some attention. However, aside from the fact that we can now say that a pharmacological agent, especially an antipsychotic, has been approved by the FDA for this indication, does it change much with regards to clinical practice, and with regards to our assessment of the role of antipsychotics in the treatment of dementia? My assessment is that it doesn’t. (Of note, while brexpiprazole is the first approval by the FDA, risperidone had been approved for short-term treatment of aggression or behavioral and psychological symptoms of dementia in some regions such as the UK, Canada, and Australia.)
Similar to aripiprazole, brexpiprazole is a partial agonist at Dopamine 2, Dopamine 3, and Serotonin 5HT1A receptors, however, it has marginally higher affinity (Ki 0.34 for aripiprazole vs Ki 0.30 for brexpiprazole) for D2 receptors and lower intrinsic activity (60% for aripiprazole vs 45% brexpiprazole; reference) which is thought to be associated with a reduced risk of akathisia. Clinical efficacy in the treatment of schizophrenia and depression is pretty similar.
While antipsychotics have previously been studied for the treatment of agitation and psychosis in dementia, prior to brexpiprazole they were not studied in large phase 3 trials intended to obtain FDA approval. Existing clinical trials show small effects on agitation compared to placebo (and minimal effects on psychosis), but despite this modest efficacy, they are pretty much the only medication group that seems to have a reliable clinical effect on agitation in dementia, so they remain widely used for this purpose, and are recommended by practice guidelines if non-pharmacological interventions have failed. A serious consideration for all antipsychotics is that they increase the risk of death in dementia, for which they have an FDA black box warning: “Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at increased risk of death.” This combination of modest efficacy, off-label use, and black box warning has been clinically tricky to navigate, and often makes patients and families alarmed as well when it comes to using antipsychotics for dementia-related problems. With brexpiprazole at least one can now say that the use is on-label, although the modest efficacy and black box warning remain.
This combination of modest efficacy, off-label use, and black box warning has been clinically tricky to navigate, and often makes patients and families alarmed as well when it comes to using antipsychotics for dementia-related problems. With brexpiprazole at least one can now say that the use is on-label, although the modest efficacy and black box warning remain.
Regarding the black box warning, the FDA label for brexpiprazole notes “REXULTI is not approved for the treatment of patients with dementia-related psychosis without agitation…,” which leads to an interesting (and unanswered) question. Does the black box warning not apply to dementia-related agitation and dementia-related psychosis with agitation? There is no mechanistic reason to think that the mortality risk with treating dementia-related psychosis would not carry over when treating dementia-related agitation. Furthermore, if the black box warning doesn’t apply to dementia-related agitation for brexpiprazole, does that mean it also shouldn’t apply to other antipsychotics when used for this purpose? (The FDA labels for other antipsychotics, aripiprazole for example, only mention dementia-related psychosis and don’t say anything about agitation.) This isn’t really a meaningful scientific question — it’s more of a mess originating from bureaucratic drug labeling, but I wonder if this will be viewed and exploited as a loophole to bypass the black box warning. Oh, the black box warning doesn’t apply because we are using it for dementia-related agitation, and not dementia-related psychosis!
Here’s how the popular medical resource UpToDate summarizes current medical thinking around antipsychotics (entry last updated on April 7, 2023):
“Antipsychotic drugs — Atypical neuroleptics have been the agents of choice for treating psychotic symptoms and agitation in patients with dementia. However, these drugs may increase mortality and are not approved for the treatment of behavioral disorders in patients with dementia by the FDA. They should not be used routinely to treat neuropsychiatric symptoms of dementia.
Nonetheless, their benefits often still outweigh their risks in patients with dementia when treatment of psychotic symptoms including hallucinations, paranoia, and delusions is critical to patient and caregiver safety, wellbeing, and quality of life. In the absence of other effective agents, we continue to advocate cautious use for severe aggression or psychosis when nonpharmacologic strategies have failed and quality of life is not being adequately addressed by other means, after informing the patients and families of the potential risks, including increased mortality.”
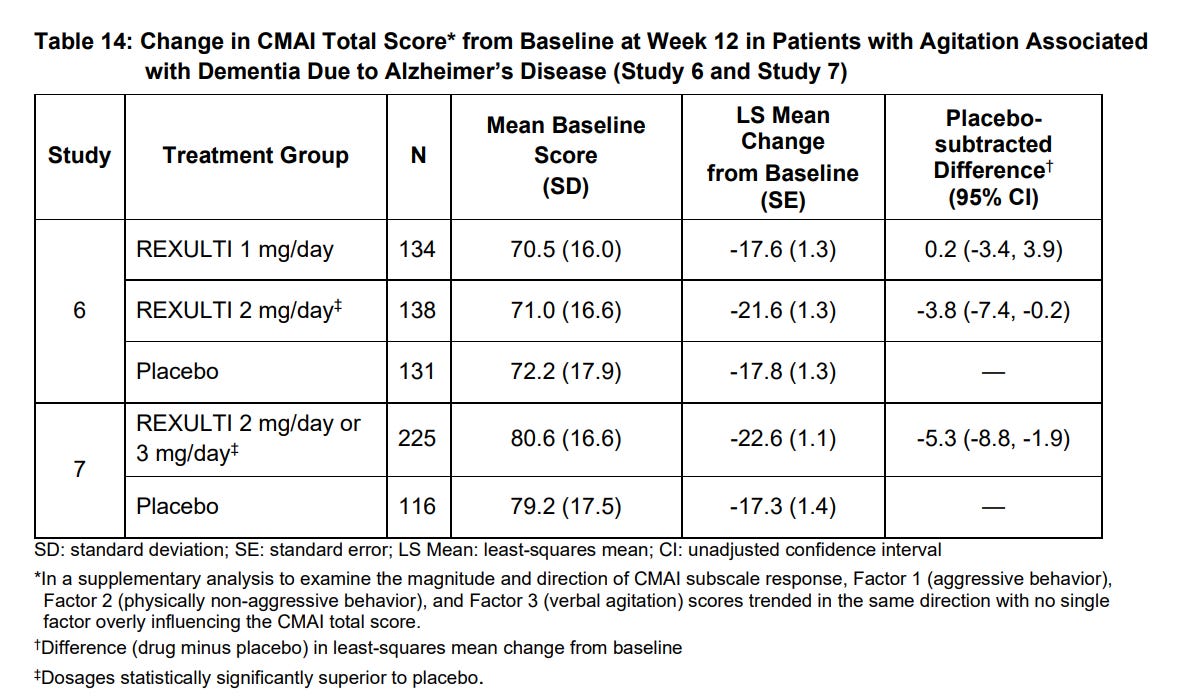
FDA approval for brexpiprazole for dementia-related agitation was based on two 12-week, randomized, double-blind, placebo-controlled, fixed-dose studies (Study 6, NCT01862640 and Study 7, NCT03548584). The primary efficacy endpoint in these two studies was the change from baseline in the Cohen-Mansfield Agitation Inventory total (CMAI) score at Week 12.
Study 7 has not been published yet in a peer-reviewed journal. Study 6 was previously published in a 2020 paper by Grossberg et al. The 2020 paper also presented the results of a brexpiprazole 0.5–2 mg/day flexible dose study in which brexpiprazole was not statistically superior to placebo, however, the subgroup that was titrated to brexpiprazole 2 mg/day had shown significance in further analyses. In the 2020 paper, brexpiprazole 2 mg fixed dose had an effect size of 0.25 and the post-hoc analysis of titration up to 2 mg in fixed dose study showed an effect size of 0.41. There were differences in effect size based on the outcome used. For example, brexpiprazole 2 mg fixed dose had effect size of 0.17 on CGI-S (Clinical Global Impression – Severity of illness) as related to agitation and 0.19 on NPI-NH [Neuropsychiatric Inventory – Nursing Home version] Agitation/Aggression domain.
Here is data from the FDA label (p32):
Let’s put this into perspective.
In a 2019 network meta-analysis, “Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia,” authors reported:
“CMAI Results: Aripiprazole (SMD, −0.30; 95% CI, −0.55 to −0.05) and risperidone (SMD, −0.26; 95% CI, −0.37 to −0.15) were associated with improvement on the CMAI compared with placebo; olanzapine and quetiapine were not”
And
“NPI Results: The NMA suggested that aripiprazole (SMD, −0.17; 95% CI, −0.31 to −0.02) was associated with improvement on the NPI compared with placebo, while olanzapine, quetiapine, and risperidone were not.”
A very blurry supplementary figure from the 2019 network meta-analysis. I have added names and SMD values above the lines to make it clear.
The performance of brexpiprazole in phase 3 trials appears to be similar to aripiprazole here.
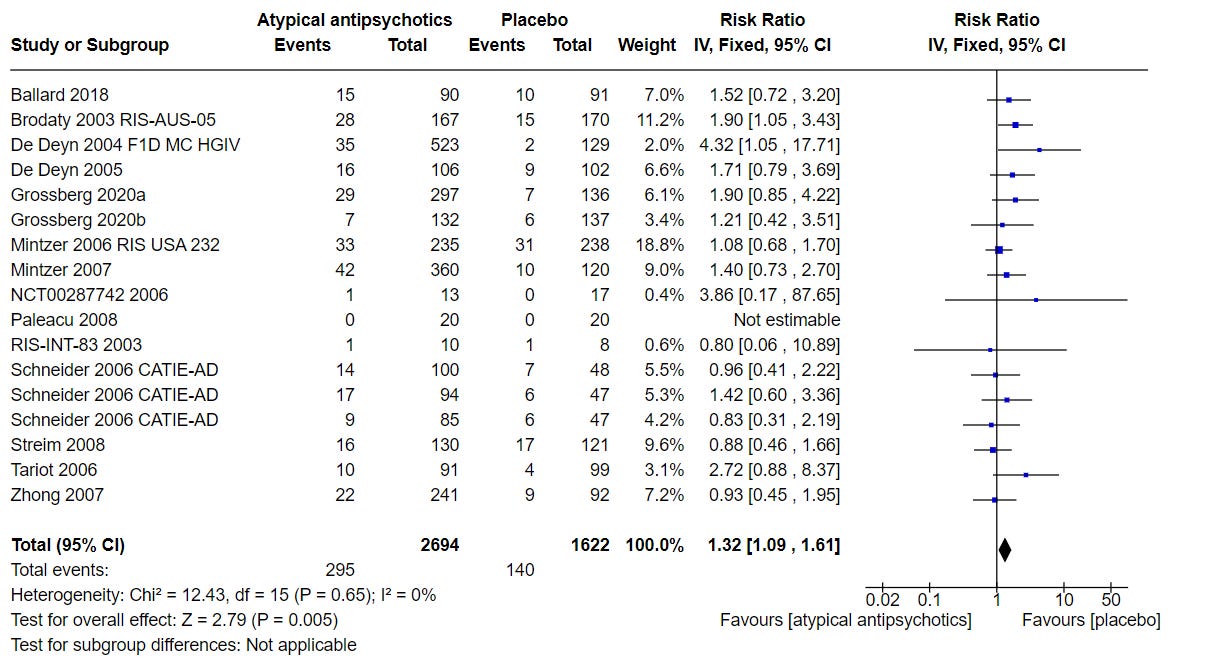
Let’s look at the 2021 Cochrane Review on the topic. Figure 34 in the paper presents a comparison of efficacy from different studies looking at agitation and aggression. Grossberg 2020 refers to the two published brexpiprazole trials. (2006 CATIE-AD looked at risperidone, olanzapine, and quetiapine.)
Based on this comparison, there is little reason to expect a substantial efficacy advantage with brexpiprazole over other agents such as aripiprazole or risperidone.
Here’s what the Cochrane review concludes about antipsychotics:
“Moderate‐certainty evidence indicates that atypical antipsychotics probably reduce agitation slightly compared with placebo (SMD ‐0.21, 95% CI ‐0.30 to ‐0.12, n = 1971). Evidence from one study indicates that atypical antipsychotic (in this study risperidone) may reduce aggression slightly as well (SMD ‐0.38, 95% CI ‐0.61 to ‐0.15, n = 301).”
Figure 39 below looks at any serious adverse event. Again, brexpiprazole doesn’t really stand out based on published data.
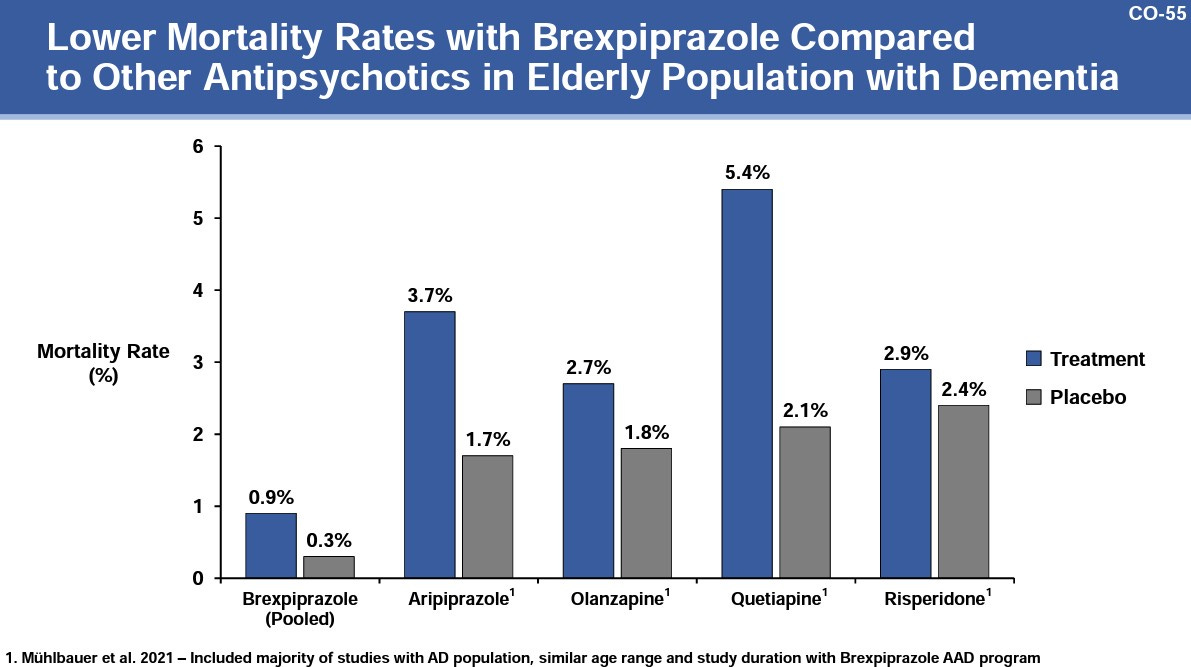
Apparently Otsuka tried to make a case to the FDA advisory committee that brexpiprazole has lower mortality rates compared to other antipsychotics (see slides 54 and 55 in this document):
But this argument is deceptive because mortality rates in the placebo comparison group for brexpiprazole are also dramatically lower vs other placebo comparison groups, and comparing relative risk ratios (for respective placebo groups) would be better here than comparing mortality rates directly. [0.9/0.3 = 3 for brexpiprazole, while 3.7/1.7 = 2.2 for aripiprazole]
On the whole, I think brexpiprazole’s FDA approval places the clinical use of antipsychotics on a comparatively firmer footing. There is little reason to think that brexpiprazole offers an efficacy or mortality advantage over other antipsychotics, nonetheless, the availability of higher quality data from phase 3 trials is something to welcome, the on-label use may be reassuring for clinicians and patients, and given the often idiosyncratic ways in which patients tolerate one antipsychotic but not another, the availability of brexpiprazole as an empirically supported treatment option is clinically valuable.
There is little reason to think that brexpiprazole offers an efficacy or mortality advantage over other antipsychotics, nonetheless, the availability of higher quality data from phase 3 trials is something to welcome, the on-label use may be reassuring for clinicians and patients, and given the often idiosyncratic ways in which patients tolerate one antipsychotic but not another, the availability of brexpiprazole as a treatment option is clinically valuable.
Bottom line: Just because brexpiprazole is the first medication, and the first antipsychotic, to receive FDA approval for the treatment of agitation associated with dementia due to Alzheimer’s Disease doesn’t mean that it has superior efficacy to other antipsychotic agents. Its efficacy appears to be similar to other antipsychotics such as aripiprazole and risperidone. Its overall safety appears similar too, especially to aripiprazole, although it is possible it may have some advantage when it comes to the risk of akathisia. Antipsychotics have modest effects on agitation in dementia, and in the absence of better pharmacological options, they can be used cautiously to treat agitation if nonpharmacological strategies have failed, taking into account their adverse effects and the increased risk of mortality.
P.S. I had already written most of the post above when I came across this tweet by Mark Oldham. Mark notes that in January, CMS announced plans to audit MDS data for inappropriate schizophrenia diagnoses (to justify use of antipsychotics in nursing homes) and adjusting star ratings accordingly, in an effort to combat off-label antipsychotic use. The Minimum Data Set—MDS—is part of the federally mandated process for clinical assessment of all residents in Medicare and Medicaid certified nursing homes. “How can brexpiprazole's approval for “agitation in AD dementia” not change the game?” He asks. Brexpiprazole is an expensive drug, currently the only on-label option, and if only brexpiprazole is considered exempt from being labeled as inappropriate and unnecessary use, it would push clinicians and facilities to use more brexpiprazole instead of other antipsychotics, which would drive up CMS costs. CMS, therefore, has a strong financial incentive, in theory, to relax its stance towards off-label use of other antipsychotics for dementia-related agitation, but will it go for it? Mark also wonders if this may create an incentive for physicians to diagnose “dementia with agitation” more often, and “dementia with psychosis” less often. Currently, dementia with agitation is often diagnosed as dementia with psychosis, unspecified psychotic disorder, or even, as CMS suspects and data indicates, schizophrenia. This trend may very well be reversed. Just another example of how systemic incentives shape nosological practices in the real world!





As someone with bipolar II, I rely on my pharmacist as much as I do my psychiatrist; I'm fortunate that both are very, very good. As someone who helps care for a person with early-onset Alzheimer's, I'm not sure this is a good idea... Here's PharmD Eric Christianson's take: "Brexpiprazole For Alzheimer’s Agitation – Is The Juice Worth The Squeeze?" https://www.meded101.com/brexpiprazole-for-alzheimers-agitation-is-the-juice-worth-the-squeeze
As someone with bipolar II, I rely on my pharmacist as much as I do my psychiatrist; I'm fortunate that both are very, very good. As someone who helps care for a person with early-onset Alzheimer's, I'm not sure this is a good idea... Here's PharmD Eric Christianson's take: "Brexpiprazole For Alzheimer’s Agitation – Is The Juice Worth The Squeeze?" https://www.meded101.com/brexpiprazole-for-alzheimers-agitation-is-the-juice-worth-the-squeeze