Schizophrenia Is the Price We Pay for Minds Poised Near the Edge of a Cliff
Cliff-edged fitness functions and the evolution of schizophrenia
This post discusses cliff-edged fitness functions by Randolph Nesse (psychiatrist and one of the founders of evolutionary medicine) and the 2024 paper, “The cliff edge model of the evolution of schizophrenia: Mathematical, epidemiological, and genetic evidence,” by Philipp Mitteroecker and Giuseppe Pierpaolo Merola.
The discussion below requires some clarifications and corrections; read them here in this follow-up.
The persistence of schizophrenia is an evolutionary enigma. It is a disabling psychiatric condition that reduces the likelihood of having children, and yet it has roughly 1% lifetime prevalence worldwide. Traditional evolutionary hypotheses, such as those invoking kin selection, mutation-selection balance, and evolutionary mismatch don’t quite explain this.
For instance, if schizophrenia risk genes were not under positive selection, we would expect them to be purged from the gene pool over thousands of years. The process will be slow, but it will happen (this is not the same as saying schizophrenia itself can be eliminated, since it is highly polygenic and the pool of risk genes is not static). Let’s say schizophrenia is associated with a 50% loss of reproductive fitness in 1% of the population (and there is no offsetting benefit in the rest of the population), then it would take roughly 180 generations to cut the rate in half and about 560 generations to reduce it to one-tenth of its original value (Mitteroecker & Merola, 2024). We’ve had a comparable number of generations since the Neolithic era, but as far as we know, the prevalence of schizophrenia has not decreased in the manner anticipated. At the same time, we do not have evidence for ongoing positive selection for schizophrenia risk alleles; in fact, schizophrenia alleles appear to be under negative selection at present, although evidence suggests many have been positively selected in the evolutionary past.
One proposed solution is to think of schizophrenia in terms of cliff-edged fitness functions, a hypothesis originally applied to schizophrenia by Randolph Nesse in 2004, and subsequently formalized by Philipp Mitteroecker and Giuseppe Pierpaolo Merola. As Nesse describes it succinctly, “I recognized that traits with an asymmetric fitness function are special; they give greater and greater advantages up to a point where the system fails and fitness falls off a cliff edge.”
In the case of schizophrenia, it can be hypothesized that certain cognitive, linguistic, or social traits increase fitness and are positively selected by evolution but lead to catastrophic consequences when expressed beyond a critical threshold. The model envisions a continuous, heritable trait that enhances reproductive fitness up to a point, beyond which it sharply decreases. Natural selection stabilizes the trait just below the cliff, but a small minority overshoot and suffer a dramatic fitness loss.
Fitness landscapes and cliff-edged functions
Biologists often rely on the metaphor of a fitness landscape to visualize how traits evolve under natural selection. Picture a three-dimensional terrain where different trait values correspond to different elevations of reproductive success. For some traits, like bird wing length, fitness peaks at an intermediate value, producing gently sloped hills. Wings that are too long or too short come with disadvantages that make birds less likely to survive environmental challenges. The population, under stabilizing selection, gravitates toward that middle ground, where fitness is maximized. This pattern extends to behavioral traits. Consider rabbits: those that take too many risks may be eaten by predators, while overly cautious ones may not forage enough to survive. The highest fitness lies in a temperate zone—moderate boldness, enough to eat but not enough to die. Natural selection nudges the population mean toward that sweet spot, but mutation and individual variability still produce outliers on either end, who fare worse as a result.
In certain cases, fitness continues to increase as a trait is pushed in one direction. Then, beyond a threshold, fitness plummets. Breeding racehorses illustrates this point. Selecting for speed led to increasingly longer, thinner leg bones. Thinner bones are also more likely to fracture. Selective breeding pushes the population of racehorses right to the edge of the cliff. The result is faster horses, yes, but also legs so fragile that a single misstep by the horse in a race can result in a deadly fracture.
Nesse hypothesizes that the same logic may apply to the evolution of human cognitive capacities. Our minds may be like the legs of racehorses. The traits that make us capable of abstract reasoning, language, and complex social cognition may—when pushed beyond a certain threshold—break down, cause severe impairment, and manifest as schizophrenia.
In cliff-edge scenarios, natural selection favors a trait value that lies just shy of the peak—not because it is optimal for everyone, but because it maximizes gene transmission across the population as a whole. A mean value where no one falls off the cliff is what would optimize health, but unfortunately for us, evolution optimizes for gene transmission. The mean value of the trait is thus set slightly below the cliff, ensuring that most individuals remain fit, but due to inevitable variation, a small fraction will fall over the edge and become ill.
Nesse (2019):
“Traits with asymmetrical fitness functions are stabilized not at the level that maximizes individual fitness (I) or at the level that maximizes health (H), but at the level that maximizes gene transmission (G), despite dire outcomes for a few individuals.” (Good Reasons for Bad Feelings: Insights from the Frontier of Evolutionary Psychiatry, p. 257)
Diseases that arise from this sort of fitness landscape would be highly heritable while being highly polygenic—arising from hundreds and thousands of common alleles with small, additive effects—that, in coordination, push individuals closer to or further from the cliff. This model fits the genetic architecture observed in complex psychiatric conditions like schizophrenia.
Examples of Cliff-Edged Fitness Functions
Many evolved traits in humans function remarkably well under typical conditions but are prone to catastrophic failure when pushed beyond narrow limits. Take brain size at birth: larger neonatal brains confer cognitive advantages, but in environments without access to modern obstetric interventions, even a slight increase in head circumference can prove fatal for both mother and infant. The margin for error is unforgiving.
The immune system operates in a similar fashion. The stakes are high and failure to mount an adequate defense can mean death. As a result, selection has shaped the immune response to be aggressive, but this same aggressiveness can misfire, turning against the body’s own tissues. Evolution optimizes for a mean value where most people benefit from an aggressive immune system and a small minority develop autoimmune conditions.
When it comes to obstructed labor, the trait under antagonistic selection is relatively straightforward: it’s the size relationship between the fetal head and the maternal birth canal. Both are polygenic and heritable, and a mismatch is deadly beyond a threshold. In contrast, “autoimmunity” doesn’t result from a single, unified trait but rather from complex interactions of a variety of processes. Still, we know that many alleles associated with autoimmune diseases confer certain immunological advantages. Some of the same genetic variants that predispose individuals to conditions like rheumatoid arthritis or multiple sclerosis may also enhance one’s ability to fight infection.
Schizophrenia is like immunocompetence in this regard: a fuzzy, composite phenotype. Increasingly, researchers consider schizophrenia to be a “meta-syndrome,” encompassing multiple symptom dimensions/clusters and arising from intersections of diverse underlying mechanisms. The absence of a single, easily measurable trait doesn’t preclude a cliff-edge dynamic. Multiple genetic variants, each with small effects, can enhance fitness through different pathways in unaffected individuals—creativity, cognitive flexibility, linguistic skill—and yet collectively increase the risk of crossing the threshold into “schizophrenia.”
What matters is not whether there's one trait under selection but whether these variants, in aggregate, follow the pattern predicted by the cliff-edge model: conferring incremental benefits across most of the distribution but leading to a sharp drop in fitness once a threshold is crossed.
What kind of positive selection magnitude is needed for cliff-edged fitness functions?
To estimate how strong positive selection would have to be to maintain such a precarious trait distribution, Mitteroecker and Merola have developed and applied a mathematical cliff edge model. They assume a heritable, continuous trait that increases fitness linearly up to a tipping point, after which fitness declines steeply. Using reasonable assumptions (1% lifetime prevalence of schizophrenia and a 50% reduction in reproductive fitness among affected individuals) they calculate the necessary selection gradient: 0.0135. That is, schizophrenia genes are very, very weakly favored by natural selection. For context, this is over ten times weaker than the average selection gradients observed in other animal traits. This suggests that schizophrenia persists due to a very weak background selection that is just strong enough to sustain the prevalence of schizophrenia without being obviously detectable.
Schizophrenia persists due to a very weak background selection that is just strong enough to sustain the prevalence of schizophrenia without being obviously detectable
Mitteroecker and Merola model a range of allele effect sizes in simulations, from slightly protective to substantially risky. When the population mean is already at an evolutionary “optimum,” even small deviations tend to reduce average fitness. In that case, most alleles—especially those with large effect sizes—are selected against. But if the population mean sits just below the optimum, then alleles with small positive effects still confer a net benefit. The model shows that selection is most favorable for alleles with moderate effects; very small effects are lost to genetic drift, and very large effects are too costly to persist.
The model predicts that most of the alleles contributing to the recent evolution of schizophrenia likely fall within an intermediate range—around 0.05 to 0.2 standard deviations in effect size. These correspond to odds ratios between 1.14 and 1.69—modest, but consistent with what we actually see in current GWAS data. Protective alleles (with odds ratios below 1) are likely smaller in effect and rarer, because they have been gradually selected against over time.
Selection pressures over evolutionary history
The selection pressures acting on schizophrenia risk alleles have not been constant over time. Early in human evolution, as cognitive and linguistic traits were expanding, the distance of the population mean was far from the cliff and no one was developing schizophrenia. As trait distributions shifted, perhaps due to cultural or ecological change, some alleles that previously conferred advantages may have pushed individuals closer to the threshold. Only when the trait distribution approached the tipping point did the first clinical cases of schizophrenia appear, triggering stronger negative selection on the most deleterious variants.
At that stage, selection dynamics likely intensified: alleles that conferred advantages (e.g., in creativity or theory of mind) continued to be selected for, while those without compensatory benefits were purged. Over time, as the population stabilized around a precarious equilibrium, even moderate-effect alleles faced increasing selective pressure. Today, many schizophrenia risk alleles may still reflect this past tug-of-war between selection for fitness-enhancing traits and the cost of overshooting.
Several studies in evolutionary genetics have explored whether schizophrenia-associated genetic loci bear signs of natural selection. Several early investigations, including both candidate gene and genome-wide approaches, have identified risk alleles that appear to have been positively selected at some point in human history. These findings are supported by converging lines of evidence.
A study by Srinivasan et al. (2016) identified a group of genes that diverged evolutionarily between modern humans and Neanderthals, many of which are implicated in brain development and function. Remarkably, more than half of those genes are also associated with schizophrenia. Genes like FOXP1 and NRXN1, which are central to language and cognitive processing, show up on this list, along with CUL3 and NRG3, which have been linked to both schizophrenia and neurodevelopmental disorders like microcephaly and intellectual disability. When these positively selected genes were analyzed for biological pathways, most of the significant hits were involved in cell-to-cell communication—particularly through cadherin signaling, which plays a key role in neural connectivity and synaptic plasticity. One gene of interest, AGBL4, sits within a so-called Human Accelerated Region (HAR)—segments of the genome that have evolved rapidly in humans and are thought to underlie uniquely human traits. Several studies now show an enrichment of schizophrenia-linked alleles in these HARs.
Other studies point toward negative selection. Large-scale genome-wide studies by Pardiñas, Yao, Liu, and González-Peñas have found evidence that many schizophrenia risk alleles are under negative or background selection.
This apparent contradiction—signals of both positive and negative selection—makes sense in light of the cliff edge model. In this framework, risk alleles can be beneficial when their effects are modest and the trait they influence stays within a certain range. But as the population-level distribution of that trait approaches the top of the cliff, natural selection begins to act against alleles with larger effects. What follows is a transition: large-effect risk alleles are increasingly selected against, while those with small effects may continue to persist.
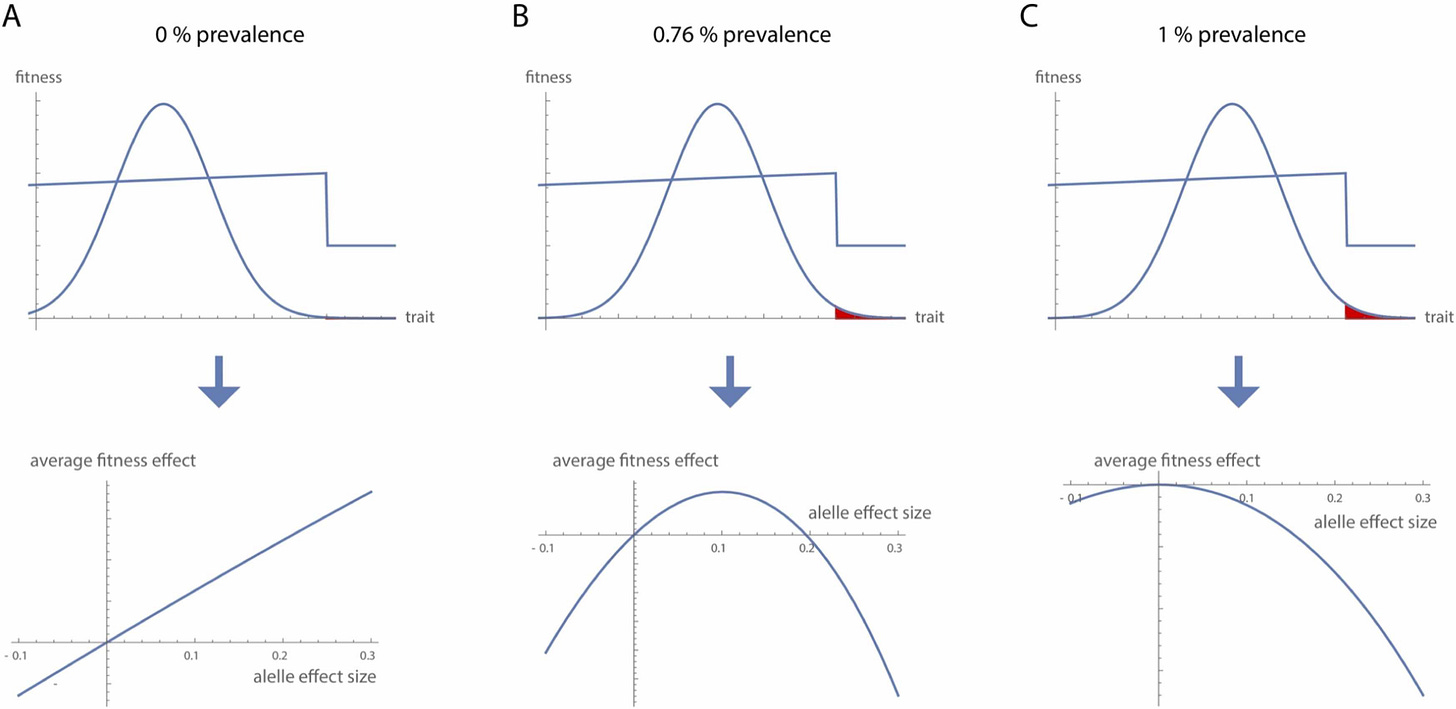
The above figure from Mitteroecker & Merola (2024) illustrates the evolutionary trajectory of schizophrenia risk alleles across three phases.
(A) In early human evolution, the distribution of the underlying trait was well below the threshold where schizophrenia emerges—far from the cliff edge. At this distance, the disorder was virtually absent, and risk alleles of all effect sizes conferred a net fitness benefit.
(B) As the population mean gradually shifted closer to the threshold, the prevalence of schizophrenia increased modestly (illustrated at 0.76%). At this stage, alleles with large effects became selectively disadvantageous, while those with smaller effects could still be maintained under weak positive selection.
(C) Eventually, the trait distribution reached an evolutionary equilibrium (here, corresponding to a 1% prevalence). At this point, all risk alleles—regardless of effect size—reduced average fitness and were subject to negative selection, as any upward shift in the trait would push more individuals over the threshold into illness.
This shifting selection landscape explains why some schizophrenia-associated alleles appear to be under positive selection while others show signs of negative selection or drift.
Do people with a higher polygenic risk score (PRS) for schizophrenia tend to have more or fewer children?
One way to empirically test whether schizophrenia risk alleles follow this kind of fitness curve is to examine how polygenic risk relates to reproductive success. Do people with a higher polygenic risk score (PRS) for schizophrenia tend to have more or fewer children?
The cliff edge model suggests they might actually have slightly more, so long as they don’t develop schizophrenia. The idea is that the same genetic variants that, in aggregate, increase the risk of schizophrenia may also enhance fitness-relevant traits like cognitive or social capacities when expressed below the pathological threshold.
This was directly tested by Escott-Price and colleagues (2019) using data from over 32,000 individuals in the UK Biobank. They computed a polygenic risk score for schizophrenia for each participant, calculated by summing the weighted effects of thousands of risk alleles. They then looked at whether this risk score was associated with the number of children participants had. In the full sample, people with higher PRS had, very slightly, more children on average. The effect was particularly evident in women, and the magnitude was in the same ballpark as that predicted by the cliff edge model.
Escott-Price et al. interpreted the observed effects as too small to explain the evolutionary persistence of schizophrenia, but Mitteroecker & Merola (2024) argue that their findings are compatible with the cliff edge model of schizophrenia, and the magnitude of a weak positive selection is also aligned with what we’d expect to see.
Genes are not the whole story, of course. The risk, severity, and outcome of schizophrenia are also shaped by a range of sociocultural and individual factors. As humans developed complex societies, it’s likely that both the prevalence and the fitness consequences of schizophrenia increased. Under such conditions, we’d expect stronger negative selection on risk alleles and stronger positive selection on protective ones, gradually shifting the population toward a new evolutionary equilibrium with lower prevalence.
Mitteroecker & Merola (2024):
“The cliff edge model, originally proposed by Nesse (2004) and formalized here, offers a compelling framework for understanding the evolutionary dynamics of schizophrenia. This model suggests that schizophrenia is the extreme manifestation of a polygenic trait or a combination of traits that, within a normal range, confers evolutionary advantages. Only beyond a certain threshold, these traits precipitate the onset of schizophrenia, leading to a dramatic drop in fitness. In this model, schizophrenia is not directly selected for, rather it emerges as a byproduct from the selection of other traits. From a genetic perspective, this implies a large set of pleiotropic risk alleles under balancing selection as they confer subtle benefits in subthreshold individuals not affected by the disorder. The actual benefits are still subject to speculation, but... many of the schizophrenia risk alleles under positive selection are known to be involved in brain development and neuronal function. Hence, it is likely that these risk alleles indeed contribute to cognitive, language, and/or social abilities.”
“An evolutionary view moves beyond the easy assumption that because severe disorders are influenced by genes, they are caused by defective genes. It calls new attention to traits and trade-offs that may result in vulnerabilities for some. The traits are unlikely to be as obvious as creativity or intelligence. Instead, they may be such factors as rates of neuron growth in early development, rates of neuron pruning in adolescence, and rates of transmission in neural networks. Tradeoffs at higher levels may also be important; for example, a tendency to attribute meaning to tiny gestures by others may be increasingly useful up to some peak—beyond which the trait crashes into sustained paranoia.
The actual systems at play are likely to be complex and difficult to identify. Nonetheless, investigating how natural selection shapes human traits to maximize fitness—but leaves some people vulnerable—suggests a way to expand the search for the keys to genetic diseases.”
See follow-up post for updates, clarifications, and corrections:
Psychiatry at the Margins is a reader-supported publication. Subscribe here.
See also:




Articles like this are the reward in the variable ratio reinforcement schedule that keep me on social media platforms. A+
This is also reminiscent of Kyaga et al's huge quantitative study on the Swedish population - turns out siblings of people with psychosis conditions (both schizophrenia and bipolar, though looks a little different for these two) are overrepresented in arts and sciences.